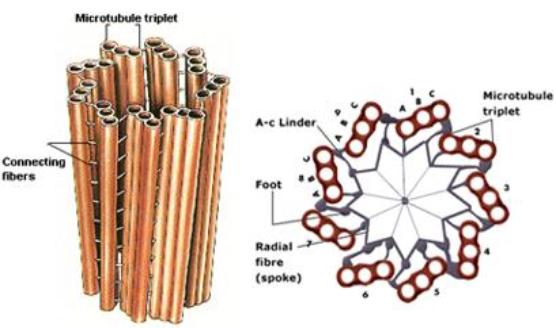


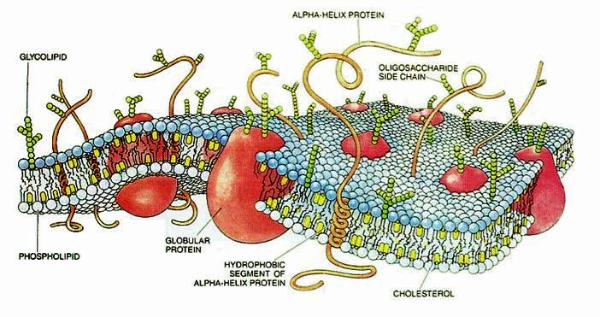
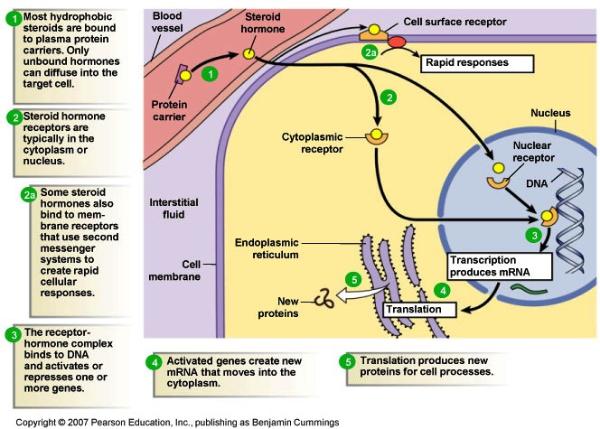
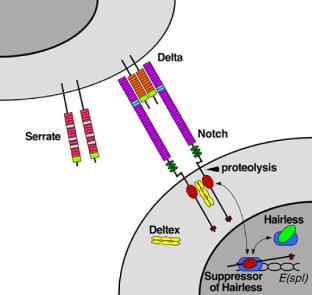
 |
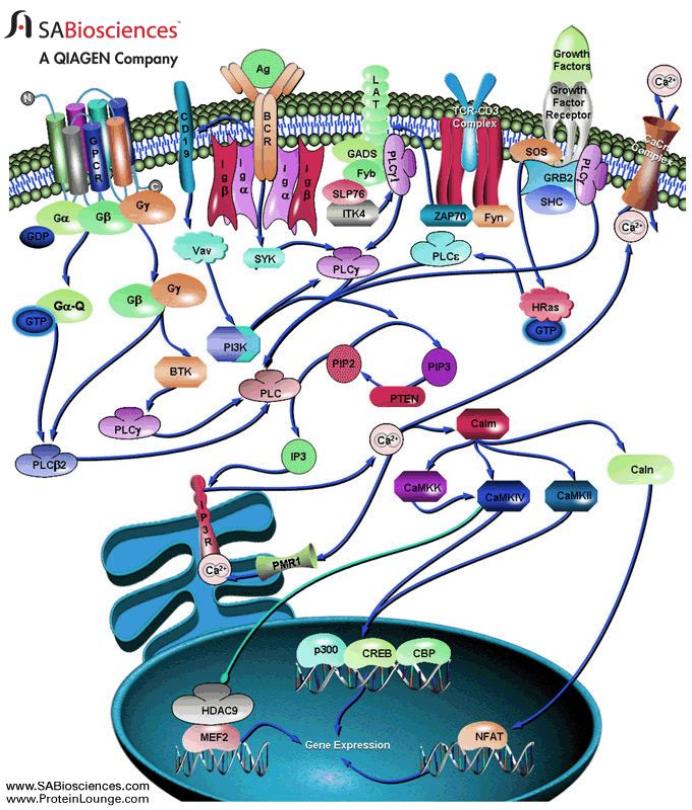
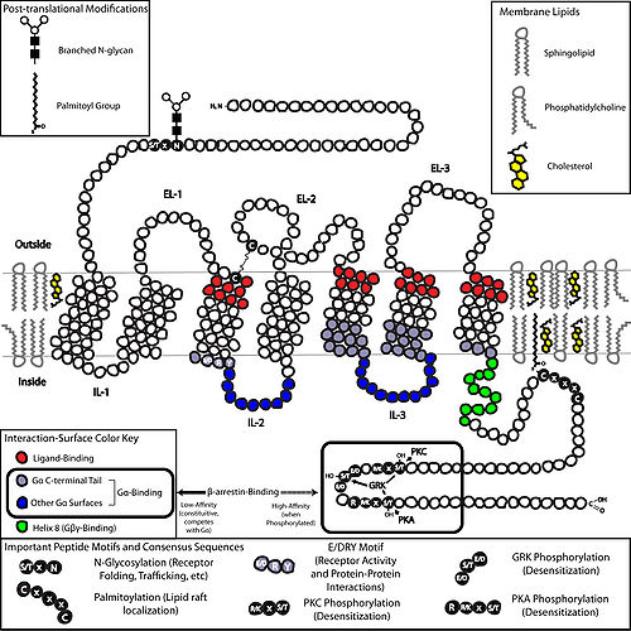
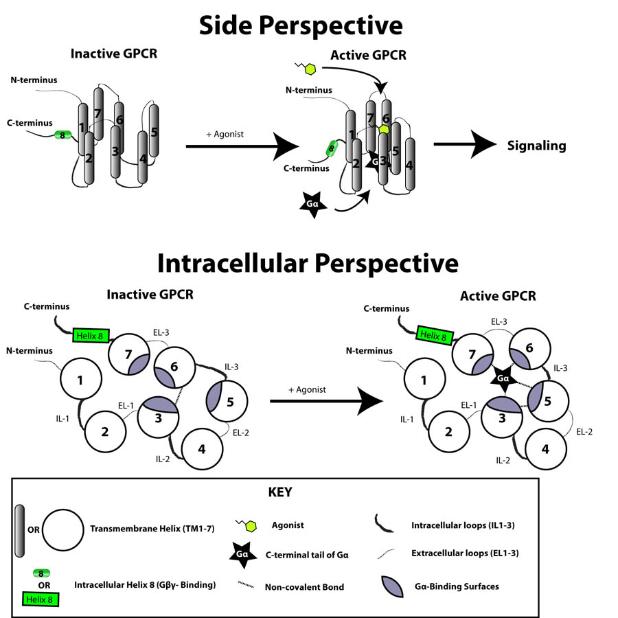
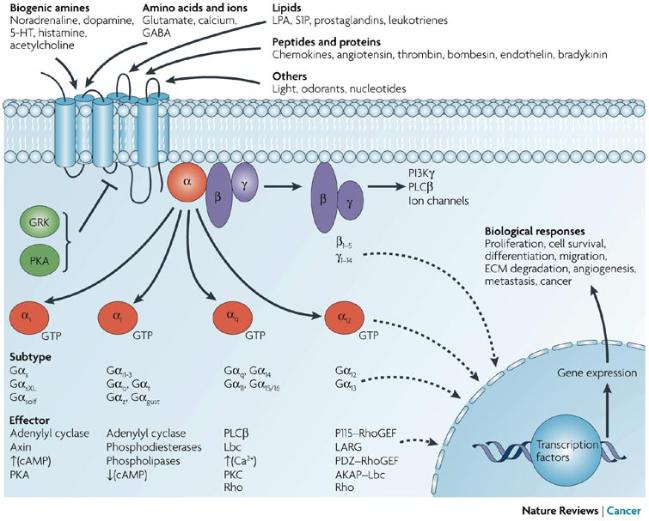
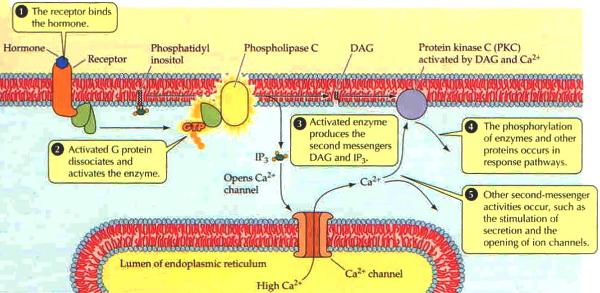
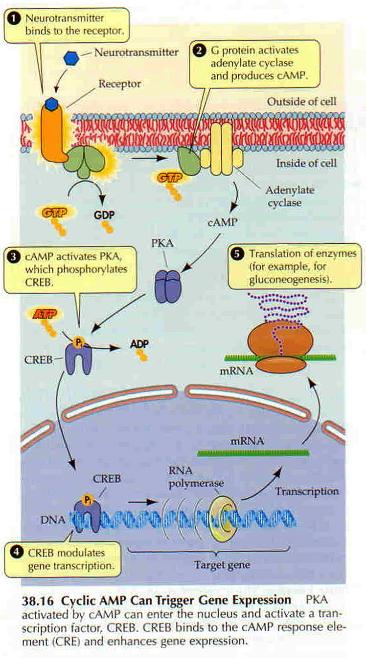
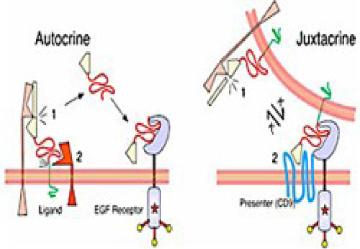
G proteins communicate signals from many hormones, neurotransmitters, and other signaling factors. The chemical signals are called ligands when they dock on a receptor. The ligand binding employs electronic forces, not covalent chemical bonds and docking is reversible. When signal molecules dock on a receptor domain outside of the cell, it causes the embedded protein receptor to alter shape in an intracellular domain that activates a "peripheral" G protein at the inner membrane. The G protein activates a cascade of further compounds, and finally causes a change downstream in the cell. G protein complexes bind to phosphate groups. G proteins (guanine nucleotide-binding protein family) respond to many signaling factors to activate signaling cascades downstream. They function as molecular switches. When attached to a complex with three phosphate groups (Guanosine triphosphate GTP), they turn on. When they are attached to a complex with only two phosphate groups (Guanosine diphosphate GDP), they turn off. G proteins regulate metabolic enzymes, ion channels, transporters, and other parts of the cell machinery, controlling transcription, motility, contractility, and secretion, which in turn regulate systemic functions such as embryonic development, learning, memory, and homeostasis. PKA is a protein kinase that is activated by cyclic adenosine mono-phosophate (cAMP). |
| Cyclic AMP (adenosine monophosphate) is a second messenger, used for intracellular signal transduction, such as transferring the effects of hormones like glucogon and adrenaline, which cannot pass through the cell membrane. It is involved in the activation of protein kinase enzymes (PKA) that regulate the effects of adrenaline and glucagon. It also regulates the passage of Ca2+ through ion channels. cAMP is synthesized from ATP on the inner side of the plasma membrane. | |
 |
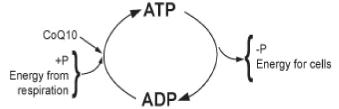 |
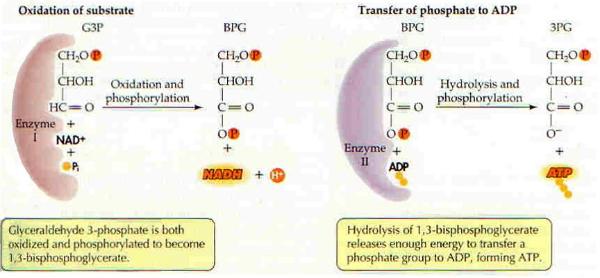
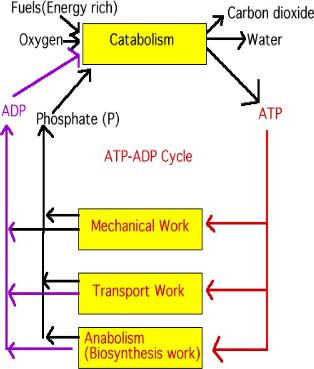
| The adding and subtracting of a phosphate to ADP is a metabolic process. Metabolic processes can be separated into two phases; catabolism is the process of breaking down (breaking down food to make ATP), and anabolism is the process of building up (using the energy created in converting ATP to ADP to build up cells or move molecules around the cell). (CoQ10 is a coenzyme.) |
 |
 |
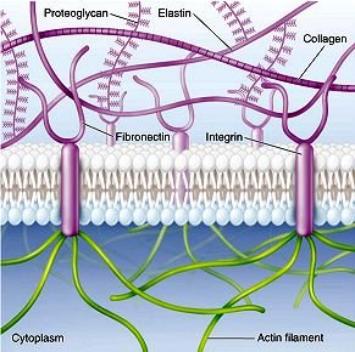
| Active transport of sodium and potassium ions (right) powers secondary active transport of glucose, amino acids, and growth factors stored in the extracellular matrix where protease enzymes break down some proteins into amino acids essential for protein synthesis in the cell. | 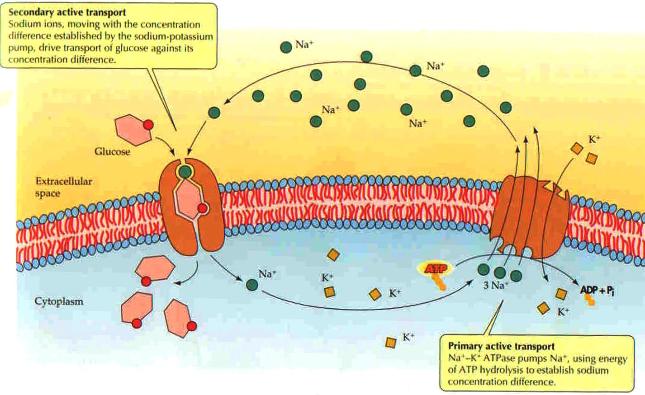 |
 |
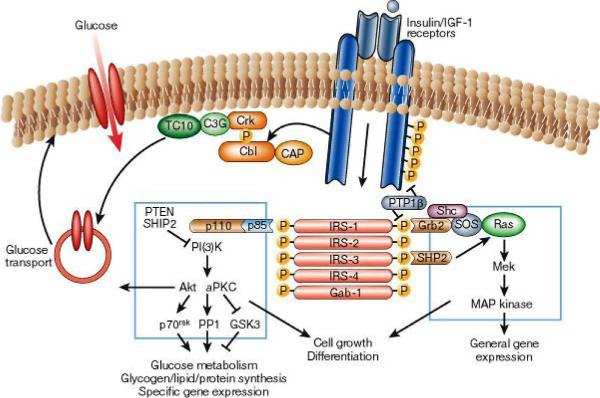
Epidermal growth factor EGF acts by binding with high affinity to epidermal growth factor receptor (EGFR) on the cell surface and stimulating the intrinsic protein-tyrosine kinase activity of the receptor to phosphorylate. This initiates a signal transduction cascade (via GRB2) that results in a variety of biochemical changes within the cell - a rise in intracellular calcium levels, increased glycolysis and protein synthesis, and increases in the expression of certain genes, including the gene for EGFR - that ultimately lead to DNA synthesis and cell proliferation. Activated Ras activates the protein kinase activity of RAF kinase. RAF kinase phosphorylates and activates MEK. MEK phosphorylates and activates a mitogen-activated protein kinase (MAPK). RAF, MEK, and MAPK are all serine/threonine selective protein kinases. In the technical sense, RAF, MEK, and MAPK are all mitogen-activated protein kinases. MAPK was originally called "microtubule-associated protein kinase" (MAPK). One of the first proteins known to be phosphorylated was a microtubule associated protein (MAP). Many additional targets for phosophorylation by MAPK were later found, and the protein was re-named. The series of kinases from RAF to MEK to MAPK is an example of a protein kinase (phosophorylation) cascade. Such series of kinases provide opportunities for feedback regulation and signal amplification. In simpler terms, the mitogen (EGF) binds to the membrane ligand. This means that Ras (a GTPase) can swap its GDP for a GTP. It can now activate MAP3K (e.g., Raf), which activates MAP2K, which activates MAPK. MAPK can now activate a transcription factor, such as myc. |
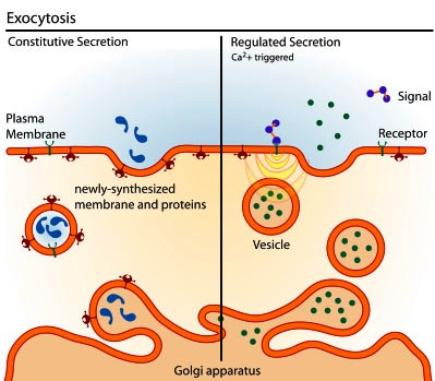 |
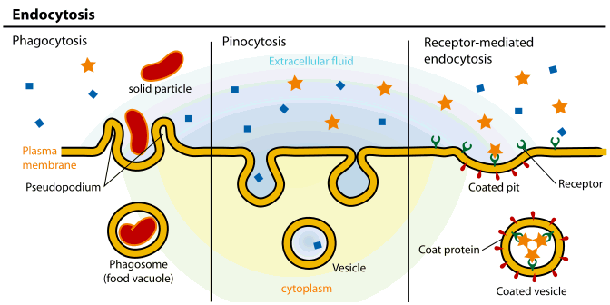
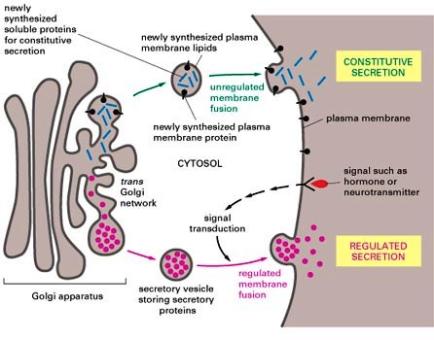
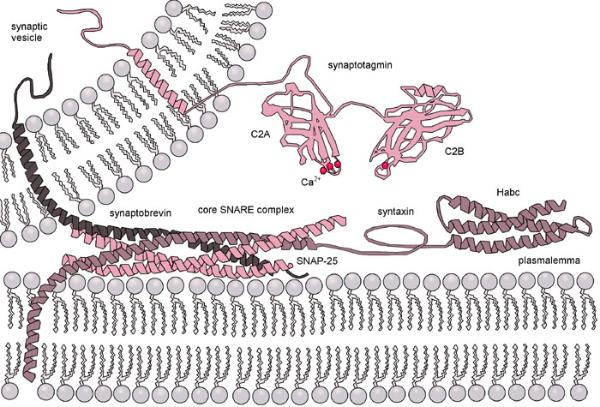
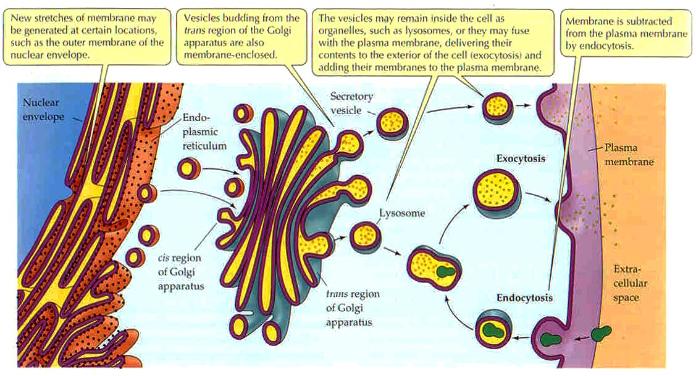
Lyosomes are produced by the Golgi Apparatus. They contain a complement of enzymes that digest the contents of phagosomes after they merge. The products, such as sugars and amino acids, diffuse from the fused lyosome for use in the cell and the waste products are removed from the cell by exocytosis. Membrane fusion requires energy and the interaction of special "adaptor" molecules present on both the vesicle and plasma membrane. The adapter molecules are highly selective and only allow vesicles to fuse with membranes of particular organelles, thus preventing harm to the cell. Once the appropriate adapter molecules bind to each other (docking), energy stored and released by ATP forms a fusion pore between the vesicle membranes and plasma membrane. The contents of the vesicle are released to the exterior of the cell (or the interior of an organelle such as a mitochondrion or another vesicle) as the fusion pore widens. The vesicle ultimately becomes part of the plasma membrane or is recycled back to the cytoplasm. Vesicle formation can be very rapid. |
 |
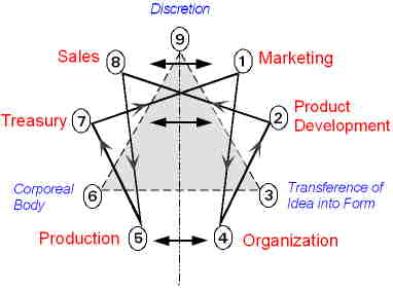 |
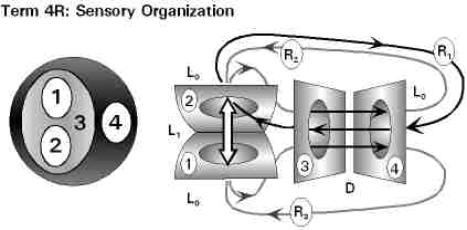
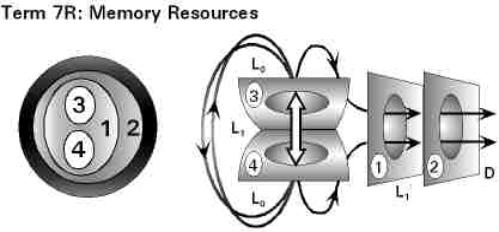
• The Particular Terms of System 4 are shown in red as they apply to a business corporation. • The flow of work transformation is shown by the connecting lines with arrows. The sequence transformations of the blue Universal Terms are not shown. • The polar insights into the Performance, Potential, and Commitment dimensions of the company are shown by the three horizontal arrows respectively. The analogy is useful lto understand cell organization and function. |