Back • Return Home
The Chemistry of Creation: Part 1 (02/12/2024)
In honor of Science Week, I wanted to take a moment to share a couple of my favorite types of chemical reactions. It might seem strange for someone to have a list of "favorite" chemical reactions, as all phenomena deserve our recognition whenever we attempt to understand the Universe around us. However, when studying life, certain things have held a fascination for me personally. Through sharing them, I hope that you will seek out the same for yourself, a collection of ideas that inspire you to do more. Science not only provides us with a method for generating knowledge that can be constructively applied towards the building of tools that can make life easier for everyone, it can also be a means for increasing our sense of wonder. We become like children whenever we ask ourselves, "How does it work?".
I will try to briefly explain the basic Chemistry needed for developing a passing familarity with each of these chemical reactions, as well as a few bits of Physics and Biology that we might encounter along the way...
...But first, what is Chemistry?
Take a quick glance around; we will see things that are made up of different materials. For example, there could be a metal bookshelf nearby, or perhaps we are sitting on a wooden chair. There is such a wide variety of materials "out there"! How do we even begin to distinguish one from another? One way is by attempting to carefully compare their properties. For example, the wood that makes up the chair is relatively soft in comparison to the metal that makes up that shelf, much like how a metal ax can chop a log of wood.
If we break things down into fine enough "pieces", we come to learn that there are about 98 naturally occuring substances within the Universe. We refer to them as "elements", because they are akin to the fundamental building blocks out of which every other substance is comprised.
Each element has its own characteristics. If there is more than one type of element within a single substance, then it is referred to as a "chemical compound". Sometimes the properties of a chemical compound are radically different from the elements that make it up. A common example is good old, Sodium Chloride, more commonly known as "table salt".
Table salt makes such a great seasoning that one might never think that it was made up of a metal that would burst into flames upon contact with water and a poisonous gas that can be fatal at fairly small dosages!
This is what a chemical reaction is, a process whereby the structure and properties of a substance change. In this case, the elements Sodium and Chlorine are referred to as "reactants". They "react" with one another to form (or "yield") a "product", Sodium Chloride. There are different types of chemical reactions, not only ones that combine (or "synthesize") elements in this way.
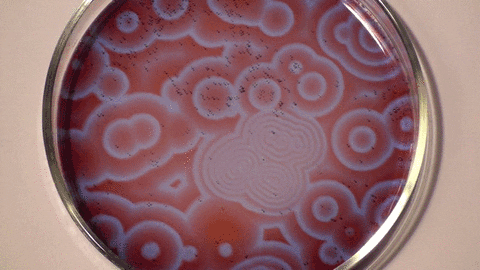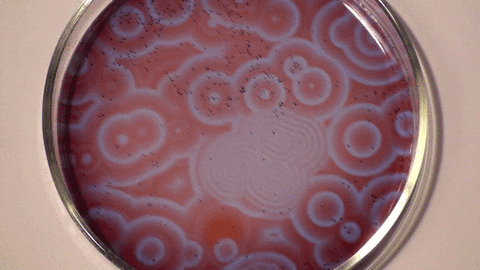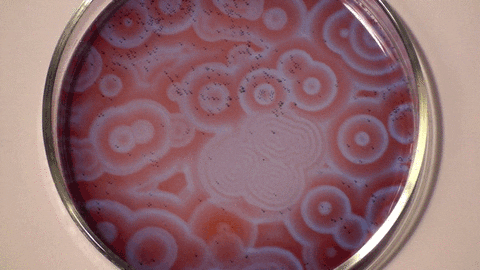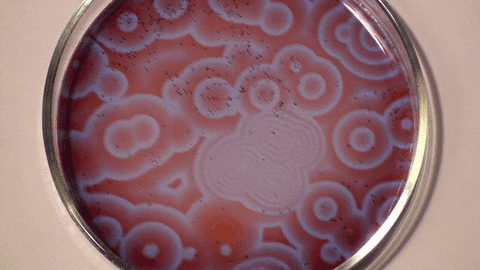
Sometimes we can speed up a chemical reaction by adding another substance. Such a substance would be known as a "catalyst", and this process as "catalysis". There are even chemical reactions where the product is its own catalyst! This is called an "autocatalytic reaction", which brings us to the first type of chemical reaction that I would like to mention: the Belousov-Zhabotinsky, or BZ reaction.
I won't dive into the details behind BZ reactions, only point out that they are a type of autocatalytic reaction that produces what is known as a "chemical oscillator" (or more informally, a "chemical clock"). This is when the amount of one or more substances periodically changes during the chemical reaction. It is fascinating to see a liquid repeatedly switch colors. And if we leave it sitting on a shallow dish, we can see little circular waves ripple throughout!
[Image from Giphy]
Notice how the waves look as if they follow "inverse square law", the same law that describes how the strength of light, gravity, and other "forces" change with the distance from their source. If we double the distance away from that source, we simultaneously halve the intensity. For example, a candle becomes dimmer the farther away we are from it. We are now moving from Chemistry into Physics.
A physicist by the name of Paul LaViolette, uses the BZ reaction as an analogy for what happens throughout space on the smallest levels of reality. In other words, a similar oscillating pattern can be used to form a model of how matter itself comes into existence. Paul refers to this model as "Subquantum Kinetics". Although his work might seem a bit strange, he is not alone in using Chemistry to describe the phenomena of Physics.
For example, Steven Rado's "Aethro-Kinematics" model attempts to use the movement of a hypothetical gas to give a mechanism behind many different phenomena, like how chemicals "bond" into compounds. It may turn out that, far from being hypothetical, these movements are carried out by an actual "substance". The work of Grigory Volovik on "superfluid" Helium comes to mind. It is interesting to think about...
Tomorrow, we will continue our exploration of autocatalytic reactions. See you then!
← Leap Into The Past • Head Into The Future →