Back • Return Home
The Chemistry of Creation: Part 2 (02/13/2024)
It is possible to have a chain of autocatalytic reactions that feed into one another, sustaining the reaction as a whole. This is known as an "autocatalytic set". These might play a significant role in the formation of "life"...Although, what is "life"?
The human body is like a laboratory overflowing with chemical reactions. In the context of living organisms, catalysts are referred to as "enzymes". Enzymes are involved in a variety of different tasks necessary to the process of living, like digesting food and building various tissues. But it is so incredibly complex, that in order to understand it, we have to simplify.
Generally, we classify compounds into two broad categories: "organic" and "inorganic". The former always have the elements Carbon and Hydrogen within them, while the latter do not (with a few exceptions). Why make this distinction? Living organisms and organic compounds tend to go hand-in-hand! As such, many scientists searched for how these substances may have appeared from seemingly "non-living" matter, a hypothesis known as "Abiogenesis".
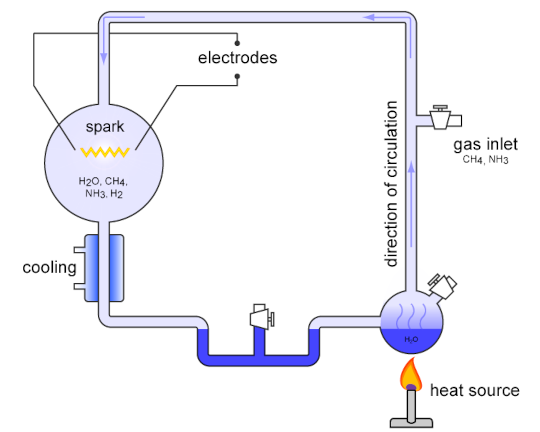
In 1952, the chemists Stanley Miller and Harold Urey conducted an experiment that attempted to mimic the conditions of a primeval Earth, with its extensive volcanic activity and severe thunderstorms. By heating up and electrifying some simple inorganic compounds (like water, methane, and ammonia), they formed organic ones!
[An image of their experimental set-up by Carny. If you have Ruffle installed, you can play around with a simulation of this experiment.]
In the following decade, the chemist Graham Cairns-Smith put forth the idea that these sorts of organic compounds were not necessarily the result of a "primordial soup" of water, methane, and ammonia existing in the Earth's oceans or atmosphere, but a process taking place on the clay of the Earth itself. Like a scientific retelling of Genesis 2:7, it turns out that clay is a fantastic medium for the creation of organic compounds. Where else might organic compounds be found?
Counter to the hypothesis of Abiogenesis is that of "Panspermia", the idea that life originated in space. Indeed, there are organic compounds in outer space that are constantly raining down upon the Earth. Some intriguing quotes that describe this process are given by the biologist Lyall Watson in his book, Lifetide: The Biology of the Unconscious. Here is one describing the so-called "stardust" that exists throughout the cosmos:
If the dust has the same composition as the interstellar gas - and there is no reason to assume otherwise - it is largely made of molecules of carbon (C), nitrogen (N), hydrogen (H), and oxygen (O). This provides an interesting, and perhaps meaningful coincidence. The grains in the interstellar medium are the same size and have the same composition as some of the most simple living things on earth, our terrestrial bacteria. Even the most complex living organisms, such as human beings, are not in essence very much different. The same four elements provide ninety-six percent of our body weight, much of it in solution. As J. B. S. Haldane observed, "Even the Archbishop of Canterbury is sixty-five percent water."
Naturally it is not enough just to have the right elements in the right proportions. Mud can do that. Something extra has to be added to breathe life into dust. New arrangements are necessary, and because carbon is one of the four basic substances, a multitude of patterns is possible.
Atoms of carbon each prefer to be linked up with four other atoms, either of their own or other kinds. And they can do so with single or double bonds, in clumps, lines, chains, branched chains, or even complete rings, to produce molecules with anything up to several million parts. These are the stuff of organic chemistry, the study of compounds producing, or produced by, living organisms; and these are the kinds of reaction which it now seems certain are taking place between the stars.
In short, stars are constantly conducting Miller-Urey-like experiments and the bodies of living organisms seem to be the beneficiaries of the material that is generated. Like the famous astronomer Carl Sagan once stated, "The cosmos is within us. We are made of star-stuff. We are a way for the universe to know itself."
However, despite being able to synthesize some fundamental building blocks of living organisms and recognize their presence under various conditions, this is still a far cry from being able to make "life". Rather than focus on the materials involved, what characteristics do living organisms have?
The biologist Robert Rosen took this kind of approach, defining life in terms of relationships. He created a model called an "(M,R) System" that tries to pin down the basic functions that an organism needs to have in order to be considered "alive". These are "metabolism" and "repair", hence an "M,R" System.
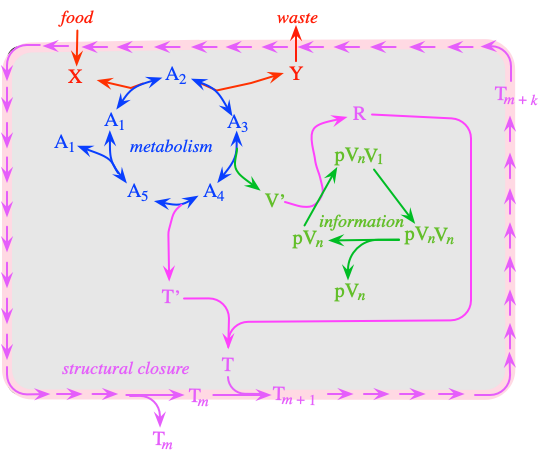
Similarly, the biologist Tibor Gánti tried to conceive of a "minimal unit" of life. He narrowed it down to three features (i.e.: metabolism, the ability to self-replicate, and the formation of a "membrane" to contain it all). He called this a "Chemoton" (short for "chemical automaton"):
[Image by Athel Cornish-Bowden. ]
The first two functions are sustained through a type of autocatalytic reaction! [A more thorough description is given within Tibor Gánti's textbook, The Principles of Life.]
Tomorrow, we will explore an aspect of these models by introducing another one of my favorite chemical reactions. Stay tuned!
← Leap Into The Past • Head Into The Future →