Back • Return Home
The Chemistry of Creation: Part 3 (02/14/2024)
A primary part of the Abiogenesis hypothesis is the formation of a "container" of some kind. By creating an internal environmentment distinct from an external one, information can be retained. In a sense, creating a container is the first step in developing a "memory"!
The structure of this container does not have to be complex. It can be formed out of simple organic compounds called "lipids". Much like how oil and water do not mix, think of a bubble of fat floating around within a body of water. Similar "protocells" might form near hydrothermal vents, fissures within the crust of the ocean floor that spew out hot water like geysers.
The layer that makes up the surface of that container (a "membrane") might let other chemicals in or out. In that case, it would be considered "semi-permeable" (i.e.: it allows some things to permeate, to pass through). Let's explore this for a moment...
If we take some table salt and mix it in a glass of water, it will eventually dissolve. This salt water mixture is generally known as a "solution". It is "homogeneous" in that it is the same throughout; we cannot see the salt crystals floating around within it anymore. The table salt is a "solute", and it is being dissolved within a larger amount of water, a "solvent". In other words, a solute is a substance dissolved within a solvent to form a solution. The amount of solute that is dissolved within a given amount of solvent is known as the "concentration" of that solution.
The direction that a solvent will flow through a semi-permeable membrane is due to the concentration of the solutions on either side of it. The solvent will move from the lower concentration to the higher concentration until both are equal. This tendency is called "osmosis". For example, if we put a cucumber in some highly salty water (a "brine"), then the water within the cucumber will flow out of it because its surface is a semi-permeable membrane. Thus, the cucumber becomes a crunchy "pickle".
If we put pressure on one of the solutions, then we can stop osmosis from occurring. This is known as "osmotic pressure". Interestingly, osmotic pressure allows inorganic chemical compounds to become convincing mimics of living organisms. A great example is the "chemical garden", first described by the alchemist Johann Glauber in 1646. However, probably one of the most amusing descriptions came some 300 years later, from the novel Doctor Faustus by Thomas Mann. To quote:
I shall never forget the sight. The vessel of crystallization was three quarters full of slightly muddy water - that is, dilute water-glass - and from the sandy bottom there strove upwards a grotesque little landscape of variously coloured growths: a confused vegetation of blue, green, and brown shoots which reminded one of algae, mushrooms, attached polyps, also moss, then mussels, fruit pods, little trees or twigs from trees, here and there of limbs. It was the most remarkable sight I ever saw, and remarkable not so much for its appearance, strange and amazing though that was, as on account of its profoundly melancholy nature. For when Father Leverkiihn asked us what we thought of it and we timidly answered him that they might be plants: "No," he replied, "they are not, they only act that way. But do not think the less of them. Precisely because they do, because they try to as hard as they can, they are worthy of all respect."
It turned out that these growths were entirely unorganic in their origin; they existed by virtue of chemicals from the apothecary's shop, the 'Blessed Messengers'. Before pouring the waterglass, Jonathan had sprinkled the sand at the bottom with various crystals; if I mistake not potassium chromate and sulphate of copper. From this sowing, as the result of a physical process called 'Osmotic pressure', there sprang the pathetic crop for which their producer at once and urgently claimed our sympathy. He showed us that these pathetic imitations of life were light-seeking, heliotropic, as science calls it. He exposed the aquarium to the sunlight, shading three sides against it, and behold, toward that one pane through which the light fell, thither straightway slanted the whole equivocal kith and kin: mushrooms, phallic polyp-stalks, little trees, algae, half-formed limbs. Indeed, they so yearned after warmth and joy that they clung to the pane and stuck fast there.
"And even so they are dead", said Jonathan, and tears came in his eyes, while Adrian, as of course I saw, was shaken with suppressed laughter. For my part, I must leave it to the reader's judgment whether that sort of thing is matter for laughter or tears.

[A beautiful chemical garden image by Tobias Kohler made from the following substances: Copper Sulphate, Iron(II) Sulphate, Iron(III) Chloride, and Calcium Chloride in Sodium Silicate "Waterglass". If you want to duplicate this same sort of experiment at home, check out this demonstration by Merchem.]
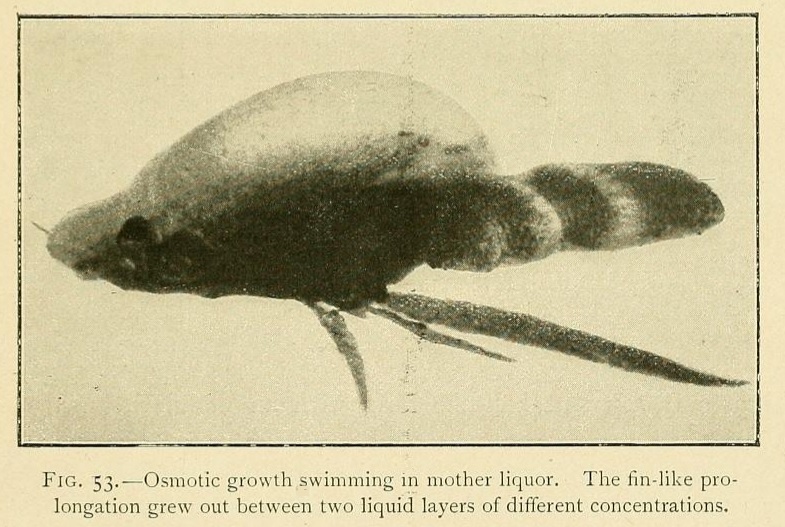
Alchemists of the early modern era produced several tree-like chemical reactions, thinking of them as evidence of life within the mineral kingdom, but chemical gardens seem like something else entirely. The biologist Stéphane Leduc referred to these types of chemical reactions as "osmotic growths", and saw them as the basis for a "Synthetic Biology" that could be developed within the laboratory. He did extensive studies of them and recorded many of his results within the book The Mechanism of Life.
Rather than the plant-like tendrils formed within the chemical garden, some osmotic growths look like other living organisms. For example, this one looks like a type of crustacean!:
But again, despite looking like life, they are completely inert. There is something missing...Perhaps energy? To quote the fascinating article, Recreating The 'Shocking' Origin Of Life In A Lab by Mary Beth Griggs:
You know that scene in Frankenstein, where the doctor uses electricity to bring the monster to life? While a work of science fiction, electricity does play a key role in all life on earth. Electrical impulses are all around us, powering just about everything we think and do. But how did the first life get that vital spark?
Back when the first life originated on Earth, the planet was a hot, unwelcome place - a mix of chemicals, rocks, and not much else. Somehow, in the midst of all this unpleasantness, something came alive for the first time. To find out how, scientists recently recreated some of these primordial conditions in the lab to find out how. They built chemical gardens, tiny replicas of the conditions at hydrothermal vents on the seafloor, where life might have originated. Like their real-world counterparts, the chemical gardens resemble chimneys rising from the depths of the sea (or the depths of the test tube).
They found that the interaction of chemicals in the lab (namely, iron sulfide and iron hydroxide) were enough to generate electricity. Linked together, four of the gardens generated enough electricity to power a lightbulb.
"These chimneys can act like electrical wires on the seafloor," lead author Laurie Barge said in a statement. "We're harnessing energy as the first life on Earth might have."
In general, there is a dynamism to electricity that seems essential to "life". When heating or electrifying a gas, we create a new state of matter referred to as a "plasma". Plasmas can form coherent, ball and ring-like structures called "plasmoids". They can also create blobs that act like living organisms! The mind swims with the possibilities, especially within the context of everything that we have mentioned so far within this series...
See you tomorrow!
← Leap Into The Past • Head Into The Future →